The visual unit: detailed analysis
Structure and function
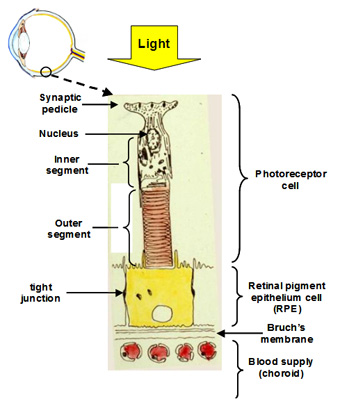
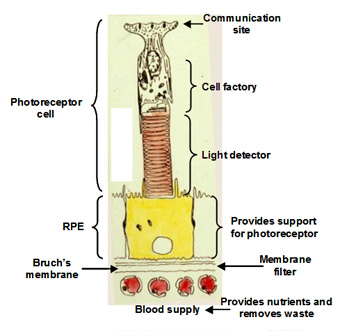
The visual unit for the detection and transmission of information comprises four compartments. The photoreceptor cell is the detector of light and communicates this information to other cells in the retina. The RPE cell provides metabolic support to the photoreceptor cell by delivering all its nutrients and removing waste products. There are tight junctions between RPE cells so that all nutrients and waste products exchanged between the photoreceptor cell and the blood supply must traverse this RPE cell. The third compartment is Bruch's membrane and this is essentially a filter that allows selective entry of blood components to be presented to the RPE for delivery to photoreceptor cells. It also serves as a barrier to the growth of blood vessels which if allowed to penetrate Bruch's would lead to the death of the photoreceptors. Finally, the fourth compartment is the choriocapillary blood supply that brings nutrients and removes waste products. The capillaries are fenestrated and therefore allow the release of most blood constituents to be presented to Bruch's membrane. The normal operation of all four compartments is essential for maintaining the structure and function of the visual process for the detection of light.
Communication with other cell types in the retina occurs at the synaptic pedicle of the photoreceptor cell. In the dark, neurotransmitters (aspartate and glutamate) are released continuously and this tells other cells in the retina that the photoreceptor is in the dark. A flash of light causes a reduction in the amount of transmitter released. The higher the intensity of light, the greater the decrease in the release of transmitters. Thus, the photoreceptor informs other cells in the retina about the presence and intensity of the light signal.
The inner segment is the power house of the cell containing regions packed with mitochondria and these produce ATP for synthetic purposes. This region of the cell is also the factory of the cell. It continuously synthesises disk membranes that are added at the junction of the inner and outer segment of the cell. As these discs are added, others at the tip of the outer segment are phagocytosed by the RPE. This synthetic capacity is so great that the whole outer segment is replaced within about 10 days, and this process is active throughout life.
The outer segment is the light detector in the photoreceptor cell. Each outer segment contains about 1000 membrane discs and embedded within the membranes is the photopigment protein rhodopsin. The detector is very sensitive such that the activation of a single rhodopsin molecule (with one photon) anywhere in the outer segment can elicit a physiological response.
The RPE performs many functions that are essential for the survival of photoreceptor cells. Firstly, the presence of tight junctions between RPE cells provides a blood-retinal barrier allowing the ionic composition around photoreceptors to be tightly regulated. Secondly, the RPE cell has protein carriers on both apical and basal surfaces that allow the delivery of essential nutrients from the blood to photoreceptor cells. Conversely, it facilitates the removal of waste products into the circulation. Thirdly, the RPE pumps fluids out of the retina and onto Bruch's for clearance into the blood. Fourthly, the RPE phagocytoses or "bites off" packets of outer segment discs, on a daily basis, and degrades these, sending the components back to the photoreceptor cell for further synthesis. The removal of discs by the RPE and synthesis in the photoreceptor is balanced so that the length of the outer segment remains constant. This process is very important in maintaining the photoreceptor cell and is discussed in more detail below.
The importance of phagocytosis
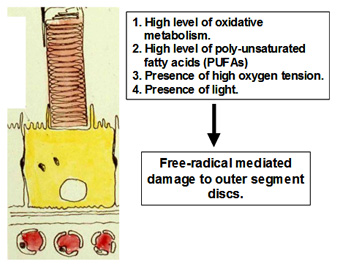
Photoreceptor cells maintain one of the highest rates of oxidative metabolism of any cell in the body. Nearly 5% of the oxygen utilised by the mitochondria is released as free radicals. High oxygen tension due to the large choroidal blood flow and presence of light also contribute to the generation of free-radicals. Since the disc membranes in the outer segment contain high levels of poly unsaturated fatty acids, they are the natural targets of free radicals. Free-radicals mediate multiple propagation reactions leading to the formation of highly complexed proteo-lipid aggregates within the disc membrane. This type of damage cannot be repaired by the photoreceptor and if allowed to accumulate, will severely handicap the receptors ability to detect light.
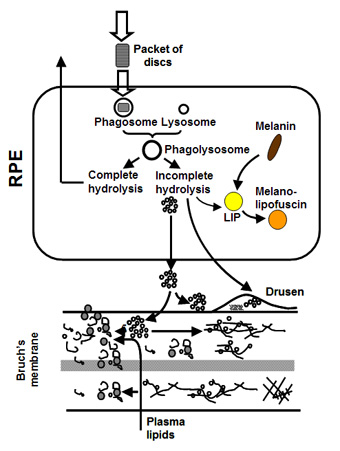
The photoreceptor sheds, on a daily cycle, packets of discs at the tip of its outer segment. This shedding is compensated by addition of newly formed discs at its base so that the length of the outer segment remains constant. Thus the severely damaged and toxic material in the discs is transferred to the RPE cell in the process of phagocytosis. The RPE attempts to break down the engulfed discs and the resulting components are recycled back to the photoreceptor. Material that is resistant to breakdown is either stored within the cell or deposited onto Bruch's membrane. The subsequent fate of this material regulates the ageing of the visual unit and is discussed in more detail below.
Ageing of the visual unit
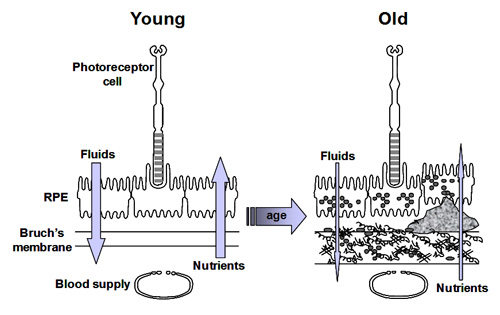
As we have stated previously, the efficient transport of fluids and nutrients across Bruch's is essential for the survival of both the RPE and photoreceptor cells.
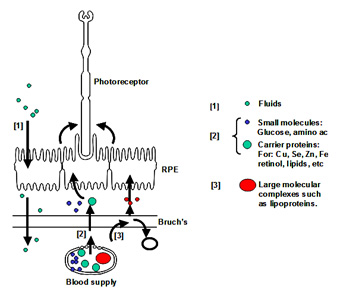
Fluid arising in the retinal layers is actively pumped out by the RPE onto Bruch's membrane. From here, the fluid is removed passively across Bruch's membrane [1] into the choroidal circulation.
Nutrients crossing Bruch's membrane can be divided into three groups. Small molecules such as glucose, amino acids, etc., exist in a free form and cross Bruch's by passive diffusion and are picked up by active/passive carriers on the basal surface of the RPE for transport to the photoreceptors. Other nutritional molecules that are normally hydrophobic or toxic in free form enter Bruch's complexed to some protein carrier. The carrier crosses Bruch's and on interaction with the RPE releases its cargo for uptake [2]. Very large molecular complexes such as the lipoproteins may partially enter Bruch's and release their cargo for further diffusion across Bruch's membrane [3]. Waste products on the other hand move in the opposite direction to be removed by the choroidal circulation.
Ageing of Bruch's and the RPE is expected to alter these transport properties. Several workers have examined the effect of ageing on the transport of fluids and nutrients and their results are briefly discussed below.
Normal turnover of Bruch's membrane
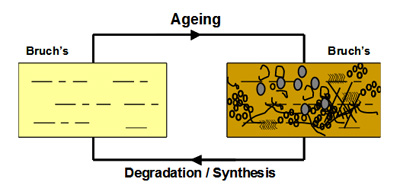
In Bruch's, a unique mechanism operates to maintain the structural and functional characteristics of the membrane. Components are continuously being broken down and replaced so as to rejuvenate the membrane. This process should therefore serve to remove the waste aggregates that become trapped in the membrane.
The degradation process is mediated by a family of calcium-containing, zinc-dependent proteolytic enzymes known as the matrix metalloproteinases (MMPs). These enzymes, upon activation, can breakdown virtually all components within the matrix of Bruch's membrane.
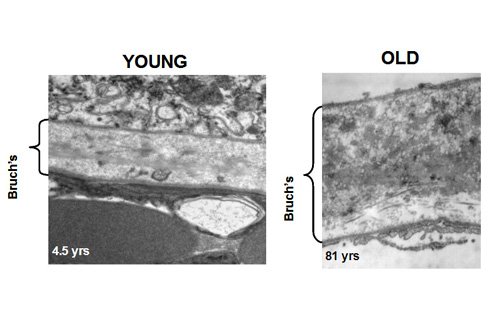
In young Bruch's membrane, the process of degradation is clearly evident since we observe the presence of activated MMPs. As we get older, this ability to degrade the matrix becomes less effective leading to the accumulation of damaged components and debris. For example, in the elderly, nearly 50% of collagen of Bruch's exists in a damaged state. There are many reasons why the degradation may be ineffective. The debris may be so damaged that the MMPs simply cannot break it down. Increasing amounts of debris may block access of the enzymes to their substrates. Furthermore, we know that some components actually inhibit the MMP enzymes.
The degradation ability slows with age but synthesis of new components continues and therefore the thickness of Bruch's increases with age. Bruch's is about 2-3 times thicker in the elderly compared to that in the young. The increased thickness, together with the deposition of debris is expected to compromise the transport pathways through Bruch's membrane
Transport pathways across Bruch's membrane
Photoreceptor cells maintain one of the highest rates of oxidative metabolism of any cell in the body. Nearly 5% of the oxygen utilised by the mitochondria is released as free radicals. High oxygen tension due to the large choroidal blood flow and presence of light also contribute to the generation of free-radicals. Since the disc membranes in the outer segment contain high levels of poly unsaturated fatty acids, they are the natural targets of free radicals. Free-radicals mediate multiple propagation reactions leading to the formation of highly complexed proteo-lipid aggregates within the disc membrane. This type of damage cannot be repaired by the photoreceptor and if allowed to accumulate, will severely handicap the receptors ability to detect light.
The photoreceptor sheds, on a daily cycle, packets of discs at the tip of its outer segment. This shedding is compensated by addition of newly formed discs at its base so that the length of the outer segment remains constant. Thus the severely damaged and toxic material in the discs is transferred to the RPE cell in the process of phagocytosis. The RPE attempts to break down the engulfed discs and the resulting components are recycled back to the photoreceptor. Material that is resistant to breakdown is either stored within the cell or deposited onto Bruch's membrane. The subsequent fate of this material regulates the ageing of the visual unit and is discussed in more detail below.
Ageing and fluid transport across Bruch's membrane
The daily fluid output by the RPE requires a hydraulic conductivity of Bruch's to be at least 0.61 x 10¯¹º m/s/Pa to be able to remove this fluid into the choroidal circulation. This is known as the failure threshold. Isolated regions of Bruch’s from many donors were evaluated as to their hydraulic conductivity. In these studies, samples were obtained from both macular and peripheral regions of the human fundus. The data given below has been adapted from: Moore et al., 1997; Starita et al., 1998; Hussain et al., 2004)
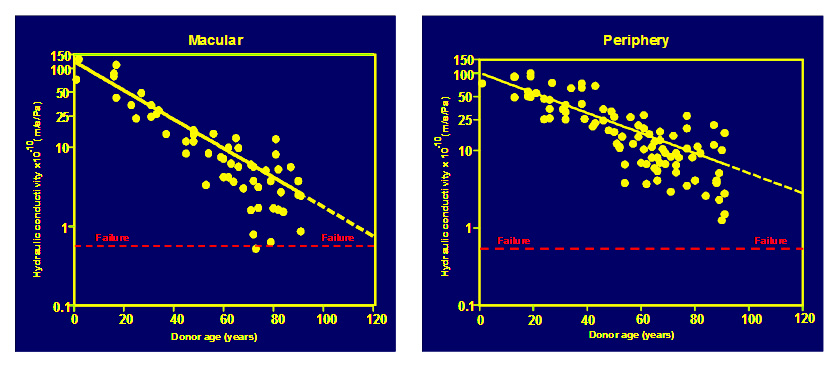
In both macular and peripheral regions, hydraulic conductivity was shown to decline exponentially with age (shown as straight lines in the semi-log plots above). The decline was much faster in the macula with a half-life of about 16 years i.e., the capacity for fluid transport by Bruch's halves for every 16 years of life. Note also that some elderly donors were very close to the failure threshold. The failure threshold and the regression line to the data in the macular samples intersects at an age of about 127 years. Hence the normal functions of Bruch's are designed to last on average of 127 years and hence most of us will not reach failure in our life times.
Ageing of fluid transport pathways in the periphery decline a little more slowly reaching failure thresholds around the age of 173 years and this may be the reason why degenerative changes are not observed in this region.
Ageing and nutrient transport across Bruch’s membrane
Most essential metals and vitamins such as vitamin A are transported in blood bound to carrier proteins. These ligand-protein complexes cross Bruch's membrane and interact with the RPE to release their payloads. Most of these carrier proteins have a radius of about 3nm. To assess the diffusional status of Bruch's membrane, FITC-dextran molecules (radius 3.3nm) were used as test probes. Donor Bruch's membrane was mounted in an Ussing chamber and the dextran molecules placed in one compartment. Diffusion of these molecules across Bruch's and into the opposite half-chamber was monitored over a period of 12 hours. The effect of age on the diffusional status of Bruch's membrane is shown below. (Data has been modified from Hussain et al., 2011).
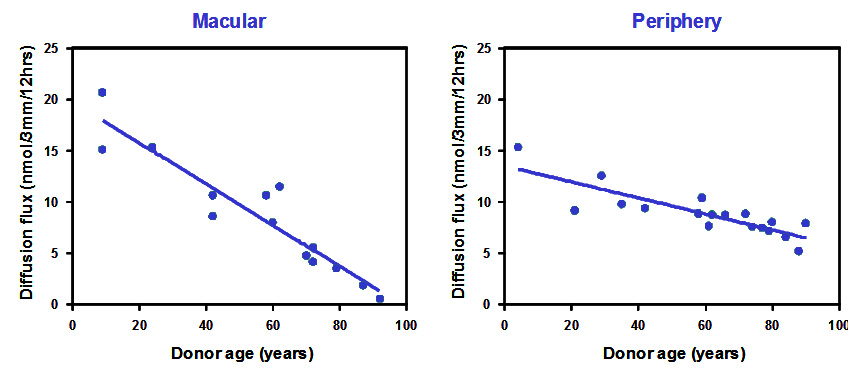
The diffusion of carrier sized test probes decreased linearly with age at both macular and peripheral locations. Macular regions showed the fastest rate of decline with some of the elderly donors approaching levels that would signify disturbance in the delivery of essential metals and vitamins. It is perhaps not surprising that elderly donors suffer from visual problems related to light and dark adaptation, processes that are dependent on the delivery of vitamin A.
Age-related Macular Degeneration (AMD) treatment depends on ‘cleaning-up’ Bruch’s membrane
Learn more about AMD
Everything you need to know about Age-related Macular Degeneration.